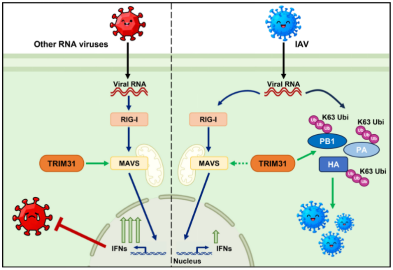
Recently, the Team for Molecular Ecology of Animal Viruses at the Lanzhou Veterinary Research Institute (LVRI), Chinese Academy of Agricultural Sciences (CAAS), launched a major advance in the study of influenza A virus (IAV) infection and immune regulation. Their latest findings, titled “The viral proteins of influenza A virus competitively bind to TRIM31 with MAVS to fine-tune the antiviral innate immunity,” have been published in the Journal of Virology. The study reveals that IAV is able to exploit TRIM31 during infection, despite its established role in activating mitochondrial antiviral signaling protein (MAVS) against RNA viruses. The viral proteins PB1, PA, and HA were found to competitively bind to TRIM31, enhancing their own stability and activity through K63-linked ubiquitination. Meanwhile, this competitive interaction attenuates the ability of TRIM31 to activate MAVS, thereby mitigating the interferon response. Through this antiviral and proviral effects of TRIM31, IAV fine-tunes the balance between antiviral responses and viral replication, maintaining the homeostasis of viral replication. The study uncovers a novel mechanism employed by IAV to adapt to host antiviral response and expands our understanding of virus-host interactions. The first author of this study is Jiaxin Huang, a jointly trained doctoral candidate from LVRI, CAAS and Gansu Agricultural University. Professors Hualan Chen at the Harbin Veterinary Research Institute, CAAS and Qiyun Zhu at LVRI, CAAS serve as the corresponding authors. In addition, associate professor Shuai Xu of LVRI and professor Caoqi Lei from Lanzhou University made a great contribution to this work. This study was supported by funding from the National Natural Science Foundation of China (U23A20243 and 32272972 to Q.Z.), Science and Technology Major Project of Gansu Province (23JRRA1513, 24JRRA806 to Q.Z.; 22ZD6NA001, 25JRRA1124 to S.X.), and Youth Innovation Program of the Chinese Academy of Agricultural Sciences (Y2023QC30 to S.X.). The full article can be accessed through the link: https://journals.asm.org/doi/10.1128/jvi.01893-25